The lube kitchen series part 8: antioxidants – the anti-ageing additive in your oil
This article has been supplied.
By: Steven Lumley - technical manager, WearCheck
(Virtual Showroom) Antioxidants is the next area of focus in the continuing discussion about oil additives, with condition monitoring specialist company, WearCheck.
Calling all pop quiz hot shots - what do blueberries, a jar of the most expensive face cream in the world and a drum of new engine oil have in common? They are all jam-packed with oxidation inhibitors, or antioxidants, as they are also known.
In this instalment of the lube series, we are going to unpack the oxidation process and explore how antioxidants keep engine oil young at heart.
Oxidation is perhaps the most common chemical reaction, not just in lubricant chemistry, but also in everyday life. Oxidation reactions take place during the combustion of fuel, when metal surfaces rust, when cleaning wounds with hydrogen peroxide and even when a cut apple turns brown.
Here’s a bit of trivia - once exposed to oxygen, enzymes in the apple begin converting natural chemicals called polyphenols into melanin, that gives the flesh a brown, rusty colour.
In a nutshell, oxidation is the loss of electrons, or the increase in oxidation state of a molecule, atom, or ion in a chemical reaction.
When it comes to lubricating oils, oxidation results in the sequential addition of oxygen to the base-oil molecules, producing a number of different chemical species, including aldehydes, ketones, hydro-peroxides and carboxylic acids.
Oxidation is the primary cause of oil degradation, and it occurs at all temperatures, but is accelerated at higher temperatures. Like many chemical reactions, oxidation rates increase exponentially with increasing temperature due to the Arrehenius rate rule. For most mineral oils, a general rule of thumb is that the rate of oxidation doubles for every 10°C increase in temperature above 75°C.
Oxidation is accelerated further by the presence of contaminants like water, and wear metals like iron and copper, which act as catalysts.
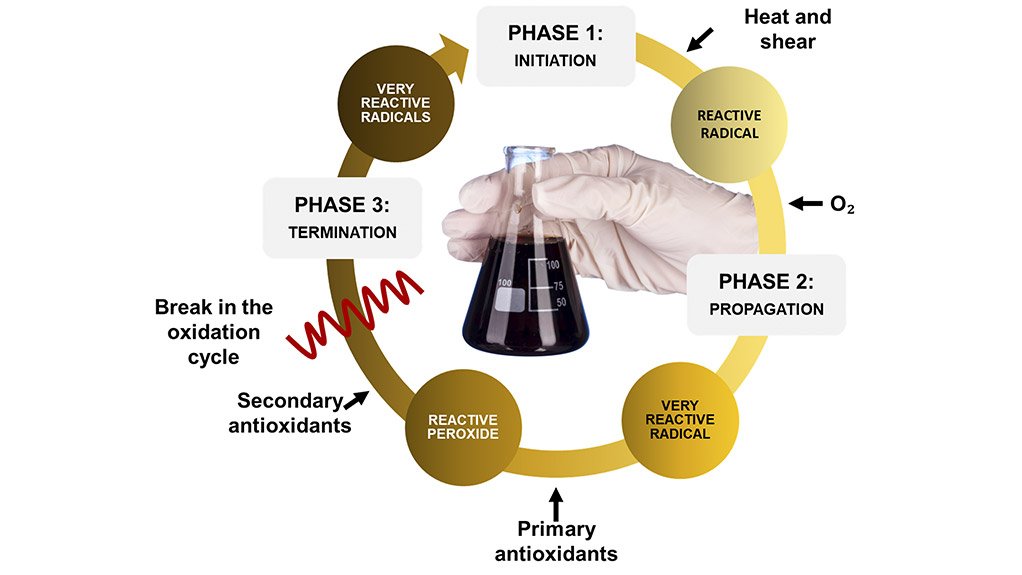
Oxidation in lubricants takes place through a complex series of chain reactions that consists of three key stages - initiation, propagation and termination.
In the initiation stage, one of the external factors (temperature, contaminants, wear metals etc) causes a free radical (or unpaired electron) to be generated in one of the organic species that is part of the lubricant. This process involves breaking a bond with a hydrogen atom.
The free radical is a highly reactive species that can react with oxygen to form a peroxide radical, that can generate additional radicals through reaction with more components in the lubricant. This step is known as propagation, and leads to further decomposition of the lubricant.
Eventually two of the radical species combine and form a stable compound. This is the termination step, because it removes free radicals from the system. However, the termination step is only effective in stopping the process if no more free radicals are formed during initiation.
In terms of its effects, oxidation typically results in impaired chemical and physical properties of the base oil and additives – increases in viscosity and organic acids, the formation of sludge and varnish, additive depletion - all of which, in turn, will have a detrimental effect on the system being lubricated.
It stands to reason that improving oxidation resistance is critical to improving lubricant stability and the operational life of the lubricant which then allows for extended oil-drain intervals.
So, now for the start of the show:
Antioxidants are used to extend the operating life of a lubricant, and these days, nearly all lubricant formulations contain some kind of oxidation inhibitor in some concentration.
They function by interrupting the three-step oxidation process, either through reaction with free radicals or decomposing peroxide radicals.
There are two main types, known as primary and secondary antioxidants. The former inhibits oxidation by reacting with chain-propagating free radicals to form stable molecules. Primary antioxidants are basically free-radical scavengers made from compounds like aromatic amines and hindered phenols.
The latter, secondary antioxidants, are peroxide decomposers that consume unstable hydrogen peroxides to form stable alcohols. It is for this reason that secondary antioxidants are also known as peroxide decomposers. These peroxide decomposers are made from phosphorus- and sulphur-containing compounds that include sulphides, phosphates and - my personal favourite - zinc dialkyldithiophosphates, or ZDDPs.
Besides functioning as an antioxidant, ZDDPs are also a very effective anti-wear agents in many applications. ZDDPs have been a mainstay of diesel-engine-oil formulation and performance for over 60 years, and with good reason - no single additive provides the same benefit as cost effectively as ZDDPs.
However, The Times They Are a-Changin’. With new engine designs and the addition of emission-control technologies, come changes to oil formulations and, needless to say, additive selection.
With the restriction in phosphorus-containing additives like ZDDPs and increased oxidation-control requirements, formulators are increasingly turning to incorporating higher levels of ashless antioxidants in their blends. Adieu ZDDPs!
Article Enquiry
Email Article
Save Article
Feedback
To advertise email advertising@creamermedia.co.za or click here
Press Office
Announcements
What's On
Subscribe to improve your user experience...
Option 1 (equivalent of R125 a month):
Receive a weekly copy of Creamer Media's Engineering News & Mining Weekly magazine
(print copy for those in South Africa and e-magazine for those outside of South Africa)
Receive daily email newsletters
Access to full search results
Access archive of magazine back copies
Access to Projects in Progress
Access to ONE Research Report of your choice in PDF format
Option 2 (equivalent of R375 a month):
All benefits from Option 1
PLUS
Access to Creamer Media's Research Channel Africa for ALL Research Reports, in PDF format, on various industrial and mining sectors
including Electricity; Water; Energy Transition; Hydrogen; Roads, Rail and Ports; Coal; Gold; Platinum; Battery Metals; etc.
Already a subscriber?
Forgotten your password?
Receive weekly copy of Creamer Media's Engineering News & Mining Weekly magazine (print copy for those in South Africa and e-magazine for those outside of South Africa)
➕
Recieve daily email newsletters
➕
Access to full search results
➕
Access archive of magazine back copies
➕
Access to Projects in Progress
➕
Access to ONE Research Report of your choice in PDF format
RESEARCH CHANNEL AFRICA
R4500 (equivalent of R375 a month)
SUBSCRIBEAll benefits from Option 1
➕
Access to Creamer Media's Research Channel Africa for ALL Research Reports on various industrial and mining sectors, in PDF format, including on:
Electricity
➕
Water
➕
Energy Transition
➕
Hydrogen
➕
Roads, Rail and Ports
➕
Coal
➕
Gold
➕
Platinum
➕
Battery Metals
➕
etc.
Receive all benefits from Option 1 or Option 2 delivered to numerous people at your company
➕
Multiple User names and Passwords for simultaneous log-ins
➕
Intranet integration access to all in your organisation